

Mesophases are of particular interest for liquid crystal technology. A phase diagram for an organic compound could include mesophases, which are intermediate phases between a solid and a liquid. A phase diagram for water might include the temperatures and pressures at which ice forms orthorhombic and hexagonal crystals. For example, a phase diagram for a substance that forms a crystal may contain lines that indicate the different possible crystal forms. Some phase diagrams contain additional information. However, these tend not to be included in phase diagrams because special conditions are required to form these phases. There are other phases of matter, such as plasma. The opposite direction, gas phase to liquid phase, is called condensation. Changing from liquid phase to gas phase is called vaporization. In the opposite direction, gas to solid phases, the material undergoes deposition. When moving between solid to gas phases, the material undergoes sublimation. When moving in the opposite direction, liquid phase to solid phase, the material is freezing. When moving from the solid phase to the liquid phase across the solid/liquid boundary, the material is melting. When the path crosses a boundary line, a phase change occurs.Įach boundary crossing has its own name depending on the direction the boundary is crossed. Phase diagrams are useful to show what will happen when the pressure or temperature moves from one point to another. The temperature where the point crosses the liquid/gas boundary is called the normal boiling point. The temperature where the point crosses the solid/liquid boundary is called the normal freezing point. These points occur when the pressure is equal to 1 atmosphere and crosses a phase boundary line. Some phase diagrams highlight two other points of interest. The minimum pressure and temperature where this occurs, Point E on this diagram, is known as the critical point. This region is known as the supercritical fluid region. Substances in this region can take on properties and behaviors of both gas and liquid. The other point of interest is when the pressure and temperature are high enough to be unable to tell the difference between the gas and liquid phases. When the material is at this pressure and temperature, it can exist in all three phases. Point D is the point where all three phases meet. There are two points of interest on a phase diagram.
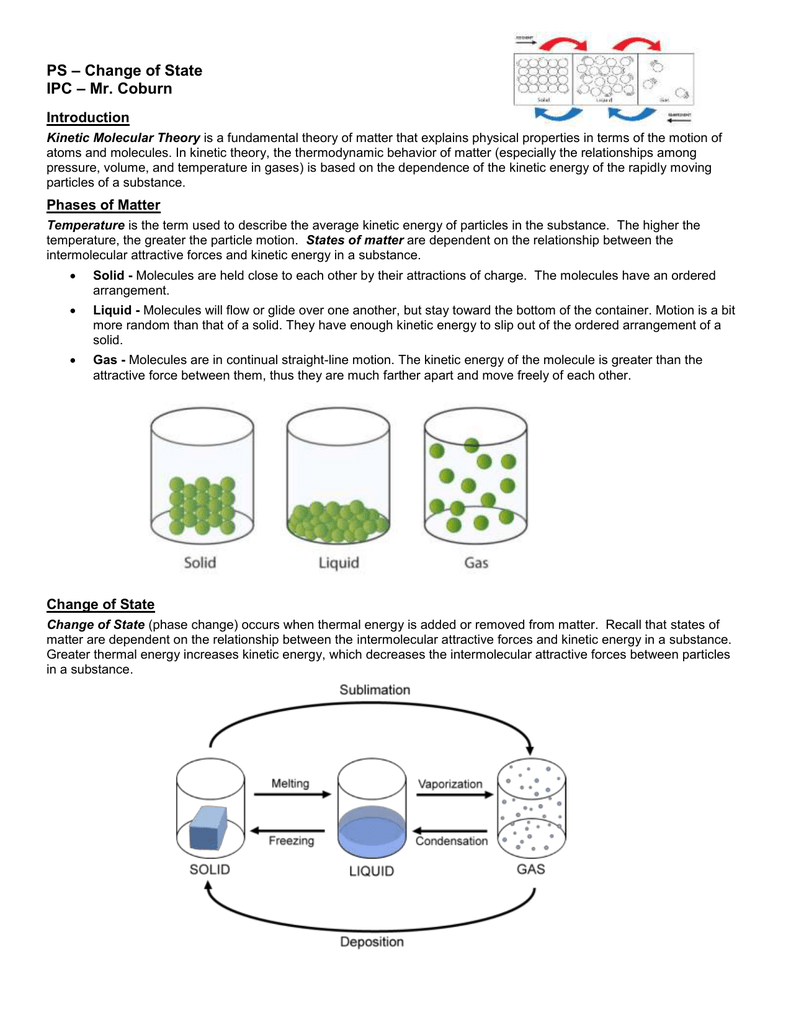
These phases exist in equilibrium with one another. At a point on a phase boundary, the substance can be in either one or the other phases that appear at either side of the boundary. These lines are known as phase boundaries. The lines on a phase diagram correspond to the dividing lines between two phases. Point B is in the liquid phase and Point C is in the gas phase. In this diagram, Point A is in the solid region. The liquid phase appears between the two regions. At low pressure and high temperature, the substance is in the gas phase. At high pressures and low temperatures, the substance is in the solid phase. States of matter include solid, liquid or gas phases. One of the properties of matter is its state.
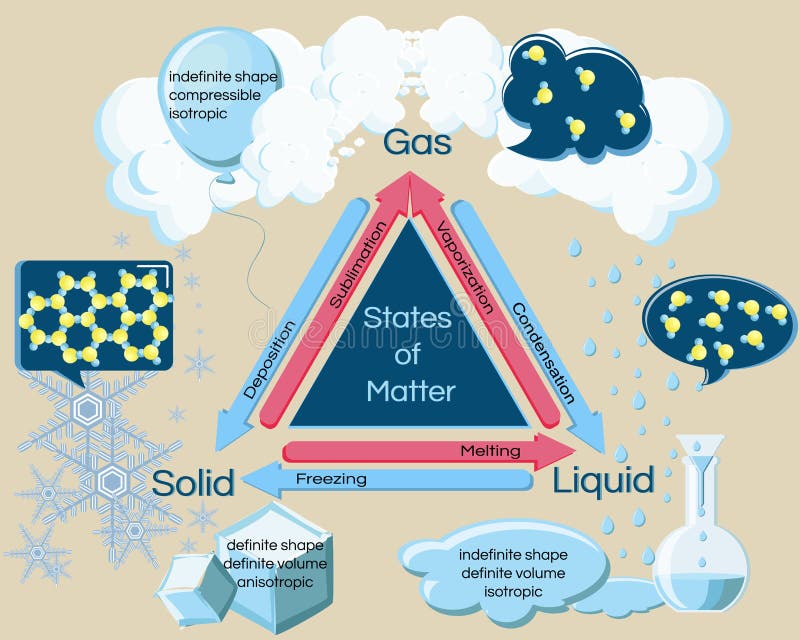
This is an example of a two dimensional phase diagram showing phase boundaries and colored coded phase regions.


 0 kommentar(er)
0 kommentar(er)
